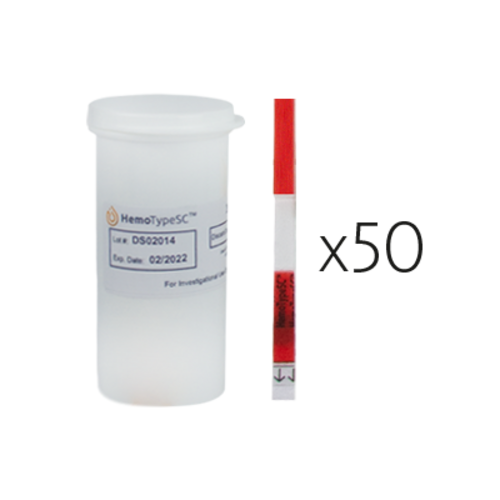
HemoTypeSC™
Universal screening for sickle cell disease and sickle cell trait
- 10 min test – no instrument required
- Reliable for neonatal and adults screening
- From capillary blood (1-2µl) or blood collection tubes (EDTA blood)
- Excellent performance: >99% clinical accuracy1,2
HemoTypeSC™ is a rapid test kit for the determination of haemoglobin type in whole blood. This is a competitive lateral flow assay incorporating monoclonal antibodies for detection of haemoglobin A, haemoglobin S and haemoglobin C.
HemoTypeSC™ provides point-of-care determination of haemoglobin phenotypes HbAA (normal), HbSS and HbSC (sickle cell disease), HbCC (haemoglobin C disease), and HbAS and HbAC (carrier or trait). HemoTypeSC™ does not detect other haemoglobin variants (such as Hb D or E) – these variants will give the same result as Hb A.
HemoTypeSC™ does not detect – and results are not affected by – fetal haemoglobin, and is therefore suitable for newborn screening.
Sickle cell disease (SCD) is a genetic condition that is present at birth. It is inherited when a child receives two sickle cell genes – one from each parent.
1 Steele et al.: Am J Hematol. 2018 Oct 5. doi: 10.1002/ajh.25305.
2 Quinn et al.: Br J Hematol. 2016 Nov; 175(4):724-73
Publications:
ADEGOKE ET AL. (2021) Hematol Transfus Cell Ther. 2021 Jan 27;S2531-1379(21)00004-3.
MUKHERJEE B ET AL. (2020) Am J Clin Pathol. 2020 Jan 1;153(1):82-87
NNODU O ET AL. (2019) Blood Cells Mol Dis. 2019 Sep; 78: 22–28.
NANKANJA R ET AL. (2019) Am J Hematol. 2019 Jun;94(6): E164-E166
| Intended use | HemoTypeSCTM is a rapid test kit for the determination of haemoglobin (Hb) type in whole blood |
| Assay principle | Competitive lateral flow assay |
| Antibody type | Monoclonal |
| Sample preparation | Whole blood |
| Sample type | EDTA, finger prick. heel stick, dried blood spots |
| Sample volume | 1.5ul |
| Storage & stability | Unopened: 15-40oC for 2 years Opened: 30 days |
| Type of result | Qualitative |
| Separate Liquid Buffer Required | No |
| Limit of Detection for Haemoglobins A, S, and C | <2%, <2%, & <2%, respectively. |
| Time to result | 5-10 minutes |
| Interference by fetal haemoglobin | No |
| Suitable for newborn screening | Yes |
| Test procedure | 2-step procedure |
| Requirement for an instrument | No |
| Sensitivity (for all phenotypes) | ≥98% in field conditions2,3,4 |
| Specificity | ≥98% in field conditions2,3,4 |
| Overall diagnostic accuracy | >98% in field conditions3 |
| Internal controls | Internal control |
| Pack size | 50 tests including 50 blood sampling devices and 3 dropper pipettes |
3 Mukherjee M, Colah R et al. Multicentre Evaluation of HemoTypeSC as a Point-of-Care Sickle Cell Disease Rapid Diagnostic test or New-borns and Adults Across India. Am J Clin Pathol. 2019; 1-6.
4 Nakanja R et al. HemoTypeSC demonstrated >99% field accuracy in a sickle cell disease screening initiative in children of south-eastern Uganda. Am J Hematol. 2019; 1-3
Sysmex West and Central Africa Ltd.
148A Marvel House, Giffard Road
Burma Camp – Trade Fair Road
East Cantonments, Accra Ghana
+233 3027 988 67
Access the training course "HemoTypeSC™ – A Rapid Point-of-Care Test for Haemoglobins That Cause Sickle Cell Disease" on the Sysmex Academy portal here.
Product documents
Regulatory Documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex![[Sysmex WCA (english)] [Sysmex WCA (english)]](/fileadmin/_processed_/9/4/csm_HemoType-01_ee46e32356.png)



![[Sysmex WCA (english)] [Sysmex WCA (english)]](/fileadmin/_processed_/9/4/csm_HemoType-01_eb8403f66e.png)