Scientific calendar June 2022
Leucoreduced blood products assure transfusion safety
For what purpose are red blood cell concentrates leucoreduced?
To extend their shelf life
To reduce adverse transfusion events and the transmission of infectious diseases
To reduce blood group mismatching
To collect white blood cells for transfusion
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
Whole blood and apheresis transfusions can be life-saving and are needed worldwide every day to a large extent. The donation, manufacturing and transfusion of blood components is a complex procedure requiring very high quality standards to sustain the health of donors and recipients.
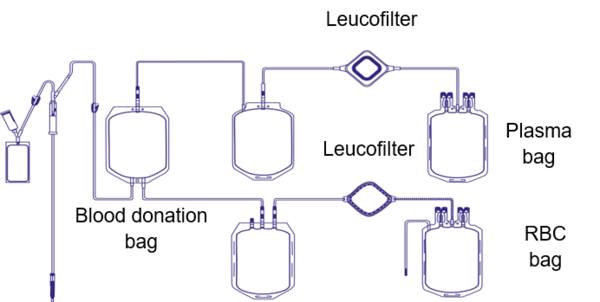
Multiple procedures are necessary before a whole blood or apheresis transfusion will be administered to a patient in need. Blood components can be plasma concentrates, red blood cell concentrates or platelet concentrates, derived either from the separation of a whole blood donation or from apheresis (a special procedure collecting only the component of interest).
One of those manufacturing steps is a process called ‘leucoreduction’ or ‘leucodepletion’, which aims to minimize the presence of the donor’s white blood cells in the blood component. The overarching intention is to reduce febrile non-haemolytic transfusion reactions or graft-versus-host disease (GvHD), and to prevent HLA alloimmunisation or the transmission of pathogens such as cytomegalovirus (CMV) [1].
By the end of the 90s and into the new century, most of the world’s industry nations established a leucoreduction step as standard procedure during blood component production. Nevertheless, various guidelines state different thresholds for quality control of the leucoreduction step. The EU directive states that more than 90% of tested blood components must contain less than 1 x 106 residual white blood cells per unit to be released for patients [2]. The white blood cells are removed by a filter which is usually attached to the blood bag used for collection.
To monitor the efficacy of the leucoreduction step, samples of the donations are assessed in regard to their content of residual white blood cells. Moreover, a check for residual red blood cells is often conducted as well (in plasma and platelet concentrates). While the main rationale for the leucoreduction is the prevention of adverse transfusion events, such as transmission of microorganisms living in white blood cells or febrile transfusion reactions, it is the reduction of potential adverse side effects due to blood group mismatching for red blood cells [3].
Recently, the transfusion of leucoreduced whole blood units became the focus of attention after a Canadian research group had shown that bacterial growth in such whole blood units does not put transfusion patients at risk [4]. This type of transfusion could be especially useful for trauma patients.
Scattergram interpretation
Patient case information
The hospitalised patient received multiple red blood cell concentrates from the hospital’s blood bank during a surgical procedure. Despite successful surgery, the patient developed fever and signs of an immune reaction hours after the surgery had been completed. A blood sample was drawn and analysed, revealing leucocytosis confirming the suspicion of an immune system activation.
Histogram and scattergram interpretation
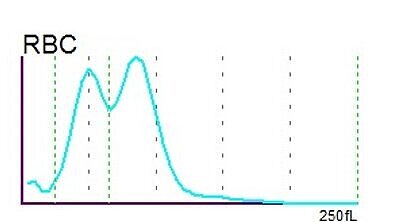
The RBC histogram from the XN-Series showed a double peak and was consequently flagged by the analyser with ‘RBC Abn distribution’ and ‘Dimorphic Population’. The double peak in the histogram is a quite common phenomenon resulting from two different RBC populations which the patient carries – the patient’s own population and the population acquired by the transfused red blood cell concentrates.
Root cause
Since the patient didn’t show any signs of infection and/or inflammation in the area of the wound, the most likely explanation was a contamination of the received red blood cell concentrates with white blood cells deriving from the blood donor.
A serologic immunoassay confirmed the patient’s infection with the cytomegalovirus (CMV), which characteristically lives in white blood cells. The patient had no history of CMV infection and hence the conclusion was that the virus had been transferred with one of the transfused red blood cell concentrates. The respective RBC concentrate was found to be contaminated with CMV-infected white blood cells of the donor.
Quality assessment of blood components
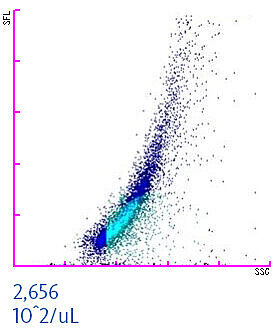
To assure the quality and safety of red blood cell concentrates (or other manufactured blood components) the success of the leucoreduction process is assessed by blood banks. When measuring a leucoreduced blood component on XN-Series analysers in Blood Bank mode, residual white and red blood cells can be quantified [3,5]. This helps in identifying contaminated transfusion units to enhance transfusion safety. The scattergram of a WBC-contaminated red blood cell concentrate is shown below. The residual white blood cell concentration should not exceed a threshold of 1 x 106 WBC per unit. In the example below, the concentration is 2,656 x102/µL. Considering that an average red blood cell unit comprises about 250 mL, this particular unit would be identified by the staff as contaminated and consequently discharged to prevent any harm to a patient.
References
[1] Mack S et al. (2020): Component residual white blood cell counting made easy? Transfusion, 60(1): 4 – 6. Free online: https://doi.org/10.1111/trf.15642
[2] European Directorate for the Quality of Medicines & HealthCare: Guide to the preparation, use and quality assurance of blood components. 19th ed. 2017, Council of Europe, Strasbourg, France.
[3] Cavagnetto C et al. (2020): Residual red cells in blood components: A multisite study of fully automated enumeration using a hematology analyzer. Transfusion, 61(2): 568 – 578. Free online: https://onlinelibrary.wiley.com/doi/full/10.1111/trf.16196
[4] Ramirez-Arcos S et al. (2022): Assessment of bacterial growth in leukoreduced cold-stored whole blood supports overnight hold at room temperature prior to filtration: A pilot study. Vox Sang. Online ahead of print.
[5] Lagerberg JW et al. (2020): Improved accuracy in counting residual white blood cells in red cell concentrates using new blood bank mode software of SYSMEX XN-1000 hematology analyzer. Transfusion, 60(10): 2456 – 2457.