Scientific Calendar December 2019
How can the immature platelet fraction (IPF) serve as biomarker for adverse cardiovascular events after surgery?
A normal platelet count with an increased IPF due to increased platelet consumption in peripheral blood may indicate increased platelet aggregation and thromboembolic complications.
Thrombocytosis with a decreased IPF due to decreased platelet production in bone marrow may indicate increased platelet aggregation and thromboembolic complications.
Thrombocytopenia with a decreased IPF due to decreased platelet consumption in peripheral blood may indicate increased platelet aggregation and thromboembolic complications.
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background information
Coronary artery diseases (CAD) are the most common cardiovascular diseases. CAD is a condition which affects the arteries suppling blood to the heart. It is usually caused by atherosclerosis, resulting in the arteries becoming narrower and in turn slowing down the blood flow.
Platelets do not adhere to non-activated endothelium. However, inflammatory events such as atherosclerosis lead to endothelial activation which stimulates platelet attachment. Platelets play a pivotal role in atherothrombosis in CAD: platelets are consumed in thrombus formation and the impact on the platelet count is compensated by an increased production of new platelets. This process of increased platelet turnover is reflected in increased immature platelet counts that can be detected in peripheral blood. Circulating levels of immature platelets are often increased in patients with CAD and several studies showed that the proportion of circulating immature platelets is associated with major adverse cardiovascular events (MACE) [1-3].
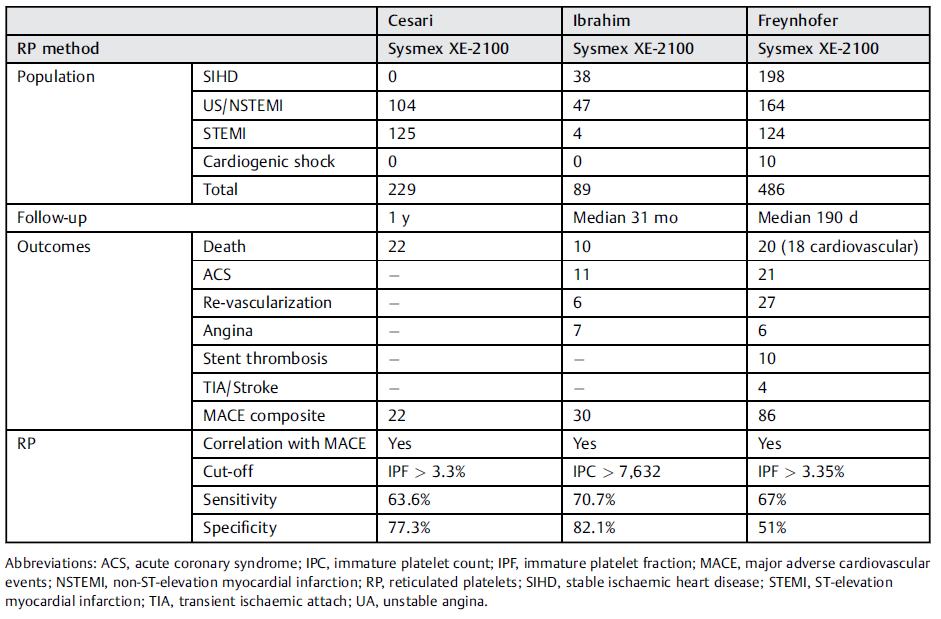
Complications after surgery require timely recognition and a consequent optimal management to improve postoperative outcome. MACE represent a frequent and particularly serious complication with a high mortality. In this context, immature platelets gained interest as a potential independent predictor for MACE [4]. The immature platelet fraction (IPF) has been shown to be elevated in patients with acute coronary syndrome [5-6] and an elevated count of immature platelets in these patients was related to an increased risk of MACE after surgery [1]. Several authors reported an association between an increased immature platelet fraction or count values and an increased risk of serious cardiovascular events [1-3]. Two of these recent studies found the same cut-off value of 3.3%, using the Sysmex IPF parameter (Table 1) [7].
Another recent study evaluated whether the immature platelet fraction (IPF) determined in the postanaesthetic care unit can predict MACE or other thromboembolic events after intermediate and high-risk non-cardiac surgery. This first study in non-cardiac surgery patients found that preoperatively, there were no differences in IPF between patients with and without MACE. On the other hand, patients with MACE had higher IPF values in the postanaesthetic care unit compared to patients without MACE. The optimal cut-off value of IPF > 5.4% was associated with an increased risk of MACE after adjustment for covariates [8]. As illustrated in this publication as well as in the scientific calendar case of this month, IPF is an independent predictor of MACE after surgery and can improve risk stratification even for non-cardiac surgery patients.
Interestingly, immature platelet reactivity as measured by light transmission aggregometry (LTA) did not predict MACE, and immature platelets seem to be better chronic predictors of adverse events than LTA, which is more likely to reflect the short-term status. Similarly, immature platelets provided a better prediction of MACE than either vasodilator-stimulated phosphoprotein phosphorylation or multiplate electrode aggregometry in patients following percutaneous coronary intervention [3].
Table
References
[1] Ibrahim H et al. (2014) Association of immature platelets with adverse cardiovascular outcomes. J Am Coll Cardiol. Nov 18-25; 64(20):2122–9.
[2] Cesari F et al. (2013) Reticulated platelets predict cardiovascular death in acute coronary syndrome patients. Insights from the AMI-Florence 2 Study. Thromb Haemost. May; 109(5):846–53.
[3] Freynhofer MK et al. (2017) Platelet Turnover Predicts Outcome after Coronary Intervention. Thromb Haemost. May 8; 117(5):923–33.
[4] Eikelboom JW et al. (2014) Immature platelet count: part of the cardiologist's complete blood count? J Am Coll Cardiol. Nov 18-25; 64(20):2130–2.
[5] Grove EL et al. (2009) Immature platelets in patients with acute coronary syndromes. Thromb Haemost. Jan; 101(1):151–6.
[6] Lakkis N et al. (2004) Reticulated platelets in acute coronary syndrome: a marker of platelet activity. J Am Coll Cardiol. Nov 16; 44(10):2091–3.
[7] Hannawi B et al. (2018) Reticulated Platelets: Changing Focus from Basics to Outcomes. Thromb Haemost. Sep; 118(9):1517–27.
[8] Anetsberger A et al. (2017) Immature platelets as a novel biomarker for adverse cardiovascular events in patients after non-cardiac surgery. Thromb Haemost. Oct 5; 117(10):1887–95.